FDA Protocol for Medication Abortion
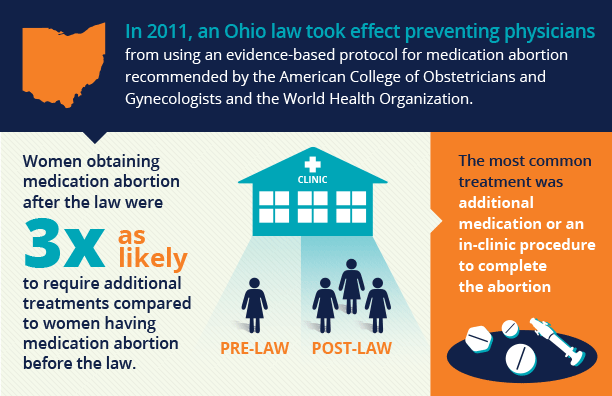
In 2011, an Ohio law took effect mandating use of the FDA-approved protocol for medication abortion. The protocol in place at that time, which was approved in 2000, restricted medication abortion to gestations ≤7 weeks LMP, used a higher, more expensive dose of mifepristone, and required an additional visit for misoprostol to be provided in clinic (see Table below). In March 2016, the FDA approved a label change for mifepristone, which brought the protocol in line with the current evidence base for medication abortion. At equivalent gestations, the evidence-based regimens have higher effectiveness rates than the outdated FDA-approved regimen. It is routinely administered throughout the U.S. and the world, and is the regimen recommended by guidelines of the American College of Obstetricians and Gynecologists, the National Abortion Federation, and the World Health Organization.
Transfer Agreements
In 2013, Ohio enacted a law requiring all abortion facilities to have transfer agreements with private hospitals. Unable to obtain such agreements, many facilities have had to close as a result.
Key Findings
- back to the Evaluation of Abortion Restrictions Project
Protocol Comparison
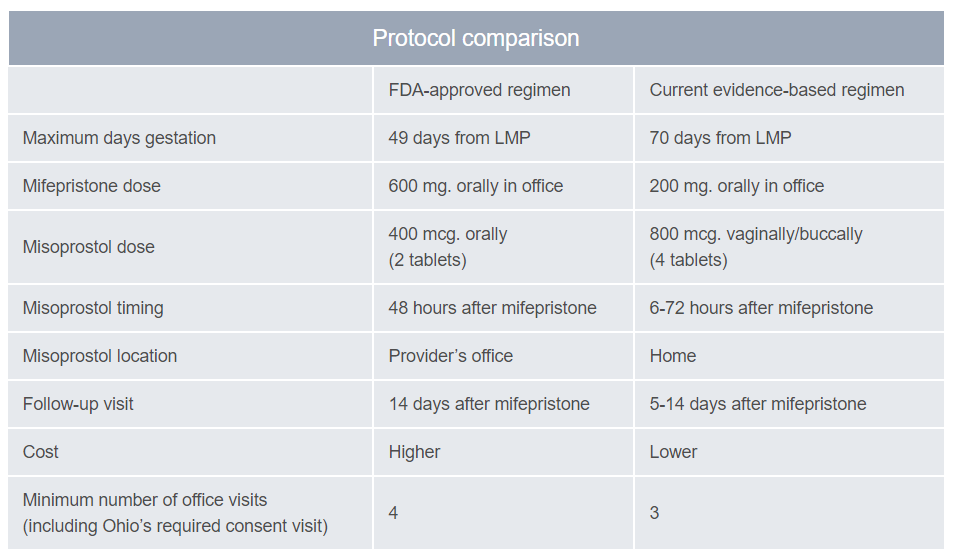
Adapted from: Reproductive Health Access Project. LMP=last menstrual period
Study Design
The Ohio FDA Protocol for Medication Abortion Study is a retrospective cohort study, comparing outcomes of medication abortions in the pre-law period to those in the post-law period. Researchers collected patient data from four Ohio abortion facilities to compare the rate of additional intervention before and after implementation of the FDA protocol and sought to understand whether implementation of the state-mandated FDA protocol in Ohio led to increases in the need for subsequent intervention among medication abortion patients; to examine changes in the sociodemographic composition of women obtaining medication abortion; and to assess the percentage of women accessing medication abortion compared to aspiration abortion prior to and following the law’s implementation.
The Ohio Transfer Agreement Study sought to understand the effects of clinic closures on women who attempt to access abortion care at a closed facility. In collaboration with providers currently at risk of closing or that have closed, we recruited women whose appointments were canceled or could not be scheduled due to the clinic closing. Women were referred to the study by clinic staff, and a phone interview was conducted with participants three weeks following their last contact with the closed clinic.